Lithium ion battery
Lithium ion battery (LIB) has been in existence since more than four decades and was largely established by John Goodenough, Stanley Whittingham and Akira Yoshino for which they won the Nobel prize in 2019 portraying its importance and impact on mankind. Especially after its commercialization in 1991, it has been scrutinized in all possible ways for its enhancement in terms of efficiency, durability, safety, portability and price. However, it still has a long way to go.
One of those attempts to improve the energy density of LIB was through increasing the mole ratios of Lithium and manganese (Li- and Mn-rich). For instance, the conception of compounds like Li1.2Ni0.13Co0.13Mn0.54O2. With increase in the lithium content, the overall capacity of the material could be increased. And with the increase in the manganese content, the price could be reduced considerably also improving the safety. Nonetheless, though such compounds provided with the high energy density, displayed poor cyclability making them practically futile. Thinking on these lines, we decided to inculcate the positives from the Li- and Mn- rich materials and at the same time find a way to increase the cyclability by tuning the band structure accordingly and hence came up with Li1.083Ni0.333Co0.083Mn0.5O2 (AS-200) a single phase cationic dominant cathode material that falls under C2/m space group.
From our analysis we realized that the voltage fade at the lower voltage was one of the main reasons for the depletion of the capacity in the Li-rich materials which was minimized in our case. A 60 mAh pouch cell was utilized in testing the material’s efficiency apart from the preliminary tests carried out in the form of coin cells. A 205 mAh/g reversible discharge capacity was attainable with more than 85% capacity retention after 400 cycles of charge/discharge.
It was interesting to note that the behavior of lattice contraction during the charge/discharge displayed by the Operando X-ray diffraction could be well-corelated to the magnetic susceptibility studies of AS-200 carried out via SQUID (superconducting quantum interference device). It was more of a novel pathway to characterize the battery material from the dynamism exhibited in their magnetic properties during the cycles. The c-lattice contraction enhanced the coupling between ions making it magnetically more ordered at low temperatures. The SQUID also revealed that the monoclinic distortion in AS-200 resulted from the strong cooperative effect on ionic size difference and specific spin alignment.
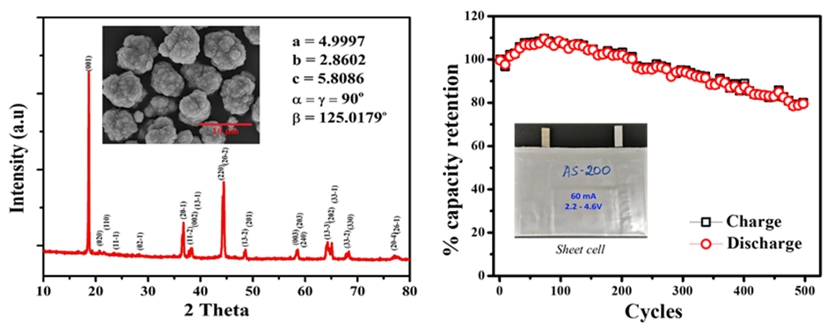
Fig1. XRD pattern and full cell cyclability of AS-200.
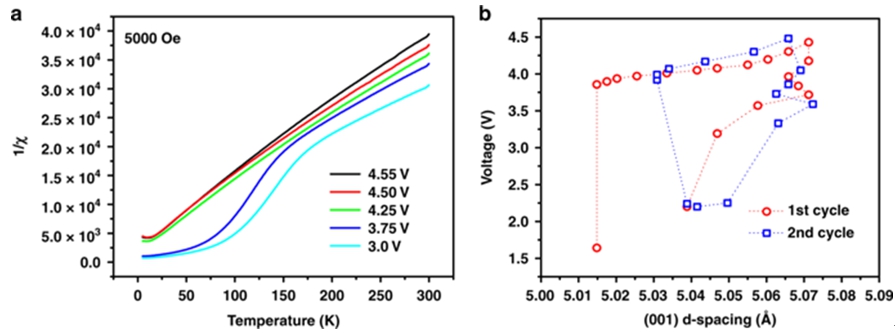
Fig2. The plot of temperature-dependent inverse susceptibility and d-spacing versus voltage for AS-200.