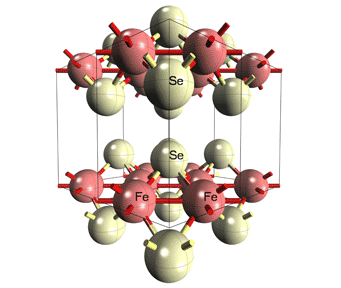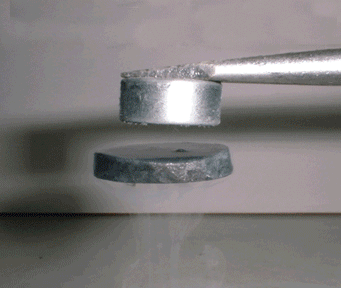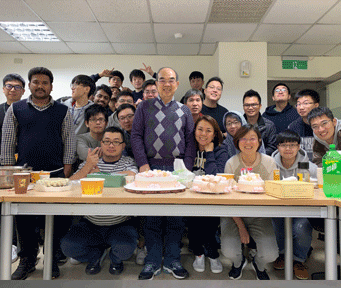
Material Anlysis
X-ray spectroscopy
Experimental Techniques
A. Synchrotron Radiation
"Synchrotron radiation" refers to a continuous band of electromagnetic spectrum including infrared, visible light, ultraviolet, and X-rays. It is emitted when a fast electron interacts with a magnetic field. When a magnetic field is applied in an area where electron is moving, it will exert a force on it perpendicular to the direction of the movement of electron. As a result, the electron will be accelerated, causing it to radiate electromagnetic energy. This is called magnetic bremsstrahlung or synchrotron radiation (since radiation is observed from particle accelerators by that name). The properties of these radiations are: (a) High Intensity (b) Continuous Spectrum (c) Excellent Collimation (d) Low Emittance? (e) Pulsed-time Structure (f) Polarization. Synchrotron radiation facilities established are very useful for the fundamental and applied research in a variety of fields in physics, biology, chemistry and crystallography, materials and geological sciences as well as medical science. You may refer the website of National Synchrotron Radiation Research Center (NSRRC) to get more information.
B. X-ray spectroscopy
It is established as a powerful tool in the analytical, physical, chemical, biological, and structural characterization of materials. For example, X-ray fluorescence is widely used in qualitative and quantitative elemental analysis; X-ray photoelectron spectroscopy (XPS) and X-ray absorption spectroscopy (XAS) can provide the electronic structure of occupied and unoccupied density of states of materials; X-ray Magnetic Circular Dichrosm (XMCD) is an extension of XPS and XAS technique in which (weak) magnetic and crystal fields are applied in the solid state, especially in 3d TM and 4f rare earth elements. The information from each of these spectroscopic techniques is unique, but all of them take advantage of the intense and continuously tunable x-rays produced at synchrotrons to identify and characterize the materials.
- X-ray photoelectron spectroscopy (XPS)
- X-ray Absorption spectroscopy (XAS)
- X-ray Magnetic Circular Dichroism (XMCD)
XPS is a surface chemical and physical analysis technique to examine electronic core-levels of the material elements; the spectra are obtained by irradiating a material with a soft X-ray beam (200-2000 eV) while simultaneously measuring the kinetic energy (KE) and number of electrons that escape from the material surface (1 to 10 nm) being analyzed. This measurement requires ultra-high vacuum (UHV) conditions (to enable the emitted photoelectrons to be analysed without interference from gas phase collisions).
| A core electron is excited into an empty valence state above the Fermi level by the absorption of an x-ray photon with energy tuned to the ionization energy of the electron (Fig. 1). When the photon energy is just sufficient to excite the electrons, a large increase in absorption occurs known as the absorption edge. This event occurs when the excitation energy of the photoelectron is not high enough to reach the ionization continuum.The XAS spectrum records the absorption intensity as a function of the incoming photon energy. The energy, intensity and polarization dependence of these resonances can be used to determine the orientation and intramolecular bond lengths of the molecule on the surface. | 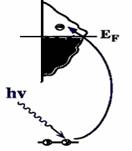 |
| Fig. 1 A core electron is excited into an empty valence state above the Fermi level. |
Several techniques exist to measure the magnetic properties of materials. Most of them are sensitive to the total magnetization of the measured system and can not discern between the contributions of different atoms in an alloy or multilayer, or between their orbital and spin moments. Moreover, the small quantity of material present in many technologically interesting samples, like magnetic nanostructures, necessitates a very sensitive measuring method. One of the most powerful techniques combining these different properties is X-ray Magnetic Circular Dichroism (XMCD). XMCD spectroscopy is an optical probe of magnetism that details the electronic and magnetic properties of the ground states of metal centers. Its foremost strengths are the element-specific, quantitative separation and determination of spin and orbital magnetic moments and their anisotropies.
Edit by Chi-Liang Chen2007/7/3