Improved Oxygen Redox Activity by High-Valent Fe and Co3+ Sites in the Perovskite LaNi1−xFe 0.5xCo 0.5xO3
For the first time Dr Raman Sankar group have successfully demonstrated a Tuning the electronic structure of perovskite oxides via aliovalent substitution is a promising strategy to attain inexpensive and efficient electro catalysts for energy conversion and storage devices. The prominent results were published in ACS Appl. Energy Mater. 2022, 5, 343−354. published by ACS on JAN 2022.
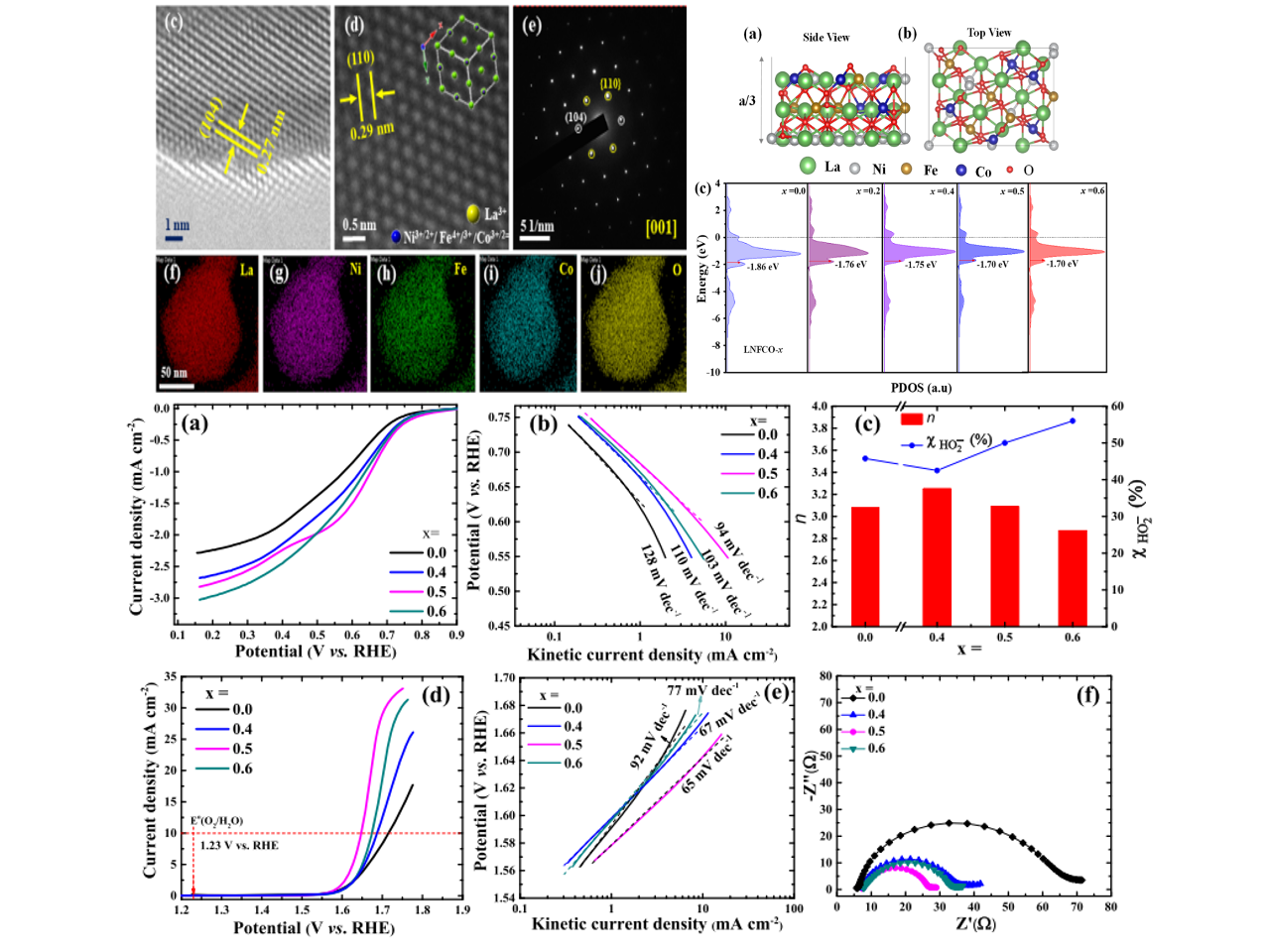
Tuning the electronic structure of perovskite oxides via aliovalent substitution is a promising strategy to attain inexpensive and efficient electrocatalysts for energy conversion and storage devices. Herein, following the d-band center positions and using a simple sol−gel method followed by a pyrolysis step, LaNi1−xFe 0.5xCo 0.5xO3 (LNFCO-x; x = 0.0, 0.4, 0.5, and 0.6) electrocatalysts are designed and synthesized for oxygen redox reactions in 1 M KOH. Among them, LNFCO-0.5 has exhibited the lowest overpotential and the highest charge transfer kinetics in oxygen redox reactions. Overall, a 90 mV lower overpotential was observed in oxygen redox activity of LNFCO-0.5 compared to that of pristine LaNiO3. The mass activity of LNFCO-0.5 in the oxygen reduction reaction (at 0.7 V vs RHE) and oxygen evolution reaction (1.60 V vs RHE) was calculated to be 2.5 and 2.13 times higher than that of LaNiO3, respectively. The bifunctionality index (potential difference between the oxygen evolution at a current density of 10 mA cm−2 and the oxygen reduction at a current density of 1 mA cm−2) of LNFCO-0.5 was found to be 0.98. The substitution of Fe and Co for the Ni-site shifted the d-band center close to the Fermi level, which can increase the binding strength of the *OH intermediate in the rate-determining step. Also, the surface was enriched with Fe3+Δ, Co3+, and partially oxidized Ni3+ states, which is susceptible to tune the eg-orbital filling for superior oxygen redox activity.
WebSite: https://pubs.acs.org/doi/pdf/10.1021/acsaem.1c02871